Only 2 out of 12 supplement companies were found to have weight-loss products that were even accurately labeled.
According to a national survey, one-third of adults who have made serious attempts at weight loss have tried using dietary supplements, for which Americans spend billions of dollars every year. Most people mistakenly thought that over-the-counter appetite suppressants, herbal products, and weight-loss supplements had to be approved for safety by a governmental agency, like the U.S. Food and Drug Administration (FDA), before being sold to the public or at least include some kind of warning on the label about potential side effects. Nearly half even thought they had to demonstrate some sort of effectiveness. None of that is true.
As I discuss in my video Friday Favorites: Are Weight Loss Supplements Safe and Effective?, the “FDA has estimated that dietary supplements cause 50,000 adverse events annually,” most commonly liver and kidney damage. Of course, prescription drugs don’t just have adverse effects; they kill more than 100,000 Americans every year. But, you at least notionally have the opportunity to parse out the risks versus benefits of prescription drugs, thanks to testing and monitoring requirements typically involving thousands of individuals.
When the manufacturer of Metabolife 356, a supplement containing ephedrine, had it tested on 35 people, only minor side effects were found, such as dry mouth, headache, and insomnia. However, once unleashed on a broad population, nearly 15,000 adverse effects were reported, including heart attacks, strokes, seizures, and deaths, before it was pulled from the market.
Given the lack of government oversight, there is no guarantee that what’s on the label is even in the bottle, as you can see in the graph below and at 1:55 in my video. FDA inspectors have found that 70 percent of supplement manufacturers violated so-called Good Manufacturing Practices, which are considered the minimum quality standards. This includes things like basic sanitation and ingredient identification. Not 7 percent in violation, but 70 percent.
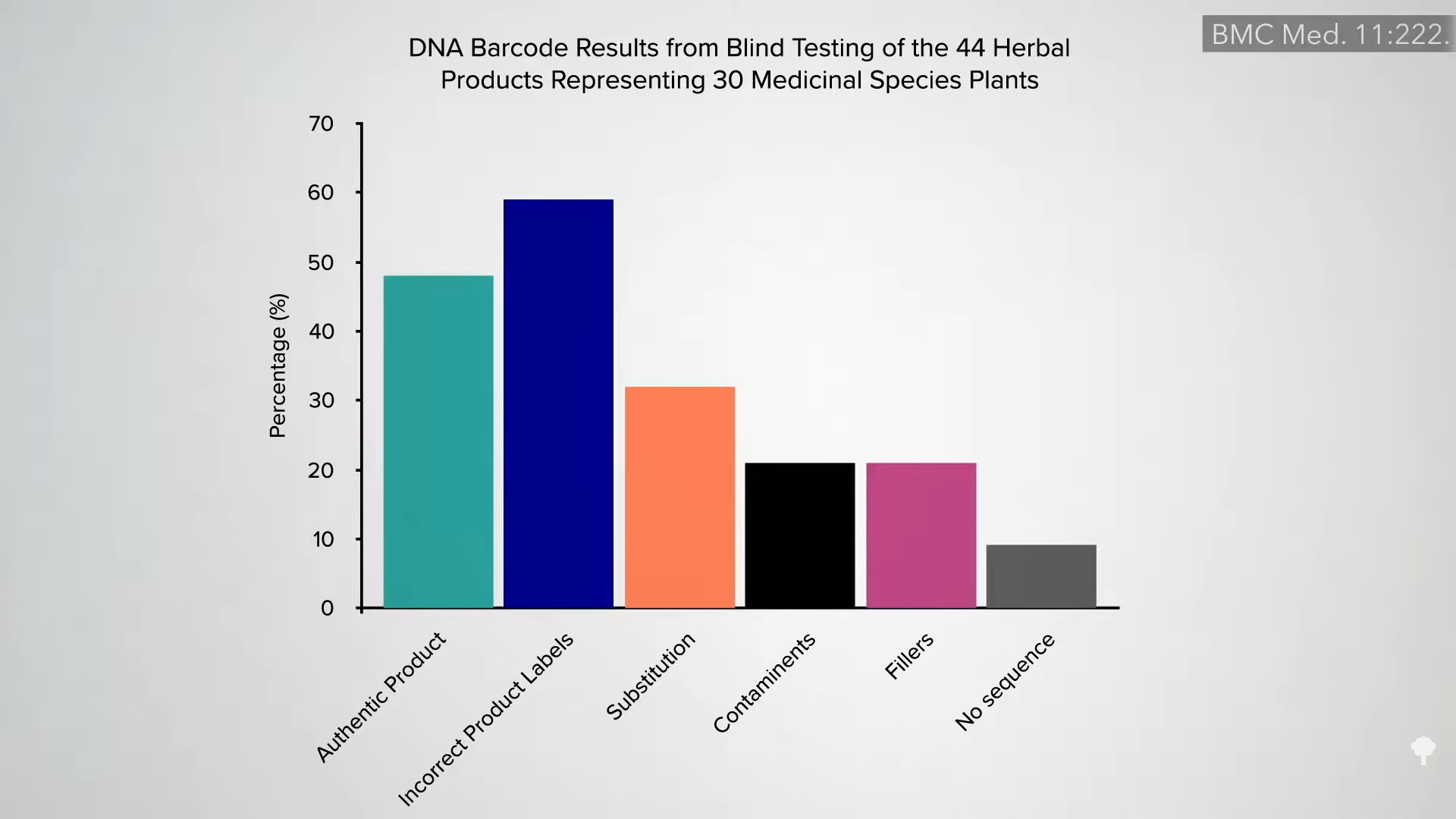
DNA testing of herbal supplements across North America found that most could not be authenticated. In a significant percentage of the supplements tested, the main labeled ingredient was missing completely and substituted with something else. For example, a so-called St. John’s wort supplement contained nothing but senna, a laxative that can cause anal blistering. Only 2 out of 12 supplement companies had products that were accurately labeled.
This problem isn’t limited to fly-by-night phonies in some dark corner of the internet either. The New York State Attorney General commissioned DNA testing of 78 bottles of commercial herbal supplements sold by Walgreens, Walmart, Target, and GNC “and found that four out of five…did not contain any of the herbs on their labels.” Instead, the capsules “often contained little more than cheap fillers like powdered rice, asparagus and houseplants…”
What about weight-loss medications? See Are Weight Loss Pills Safe? and Are Weight Loss Pills Effective?. Also, see related posts below.
Take a deep dive into the best way to lose weight with my book How Not to Diet.